 Pharmaceutical production entails strict quality control and precise analytical testing methods. The United States Pharmacopeia (USP), European Pharmacopeia (EP) and Japanese Pharmacopeia (JP) have promoted TOC analysis as the analytical technique to verify that cleaning validation, purified water (PW) and water for intravenous injection (WFI) meet the high standards of the pharmaceutical industry.
Pharmaceutical production entails strict quality control and precise analytical testing methods. The United States Pharmacopeia (USP), European Pharmacopeia (EP) and Japanese Pharmacopeia (JP) have promoted TOC analysis as the analytical technique to verify that cleaning validation, purified water (PW) and water for intravenous injection (WFI) meet the high standards of the pharmaceutical industry.
TOC analysis is an indirect means to ensure cleanliness of the water used in manufacture of pharmaceutical products and the manufacturing equipment that produces them. TOC is a useful “catch all” cleaning validation procedure for major manufacturing contaminants because the presence of carbon is indicative of a variety of cross-contamination including cleaning agents, foreign materials (paint, hair, building materials, etc.) and bacteria.
Required Validations in the Pharmaceutical Industry
- Cleaning Validation - Cleaning validation procedures verify that pharmaceutical and cosmetic manufacturing equipment that has been cleaned using clean-in-place (CIP) cleaning procedures is contamination free prior to initiating the next production run. Contamination could diminish the safety, strength, quality or purity of the product. Refer to AN1102 - A Cleaning Validation Swab Recovery Study Using a UV/Persulfate Analyzer by clicking here.
- PW and WFI Validation - WFI is water that is intended for use in the manufacture of injectable drugs. Both PW and WFI are vital to drug preparation and must be in the ppb or even sub-ppb carbon range. Refer to our application note AN2204 - Fusion USP 643 Bulk and Sterile Water Testing.
Validation Techniques in the Pharmaceutical Industry
There are several common validation techniques used in the pharmaceutical industry including:
- Thin Layer Chromatography (TLC)
- High Pressure Liquid Chromatography (HPLC)
- Spectrophotometric
- Conductivity
- Total Organic Carbon (TOC)
Table I shows a comparison of validation techniques and the types of validation in which they are used. An overview of each technique follows.
|
Table I Comparison of Pharmaceutical Validation Techniques |
|||||
|
Method |
Residual Drugs |
Cleaning Agents |
Excipient1 |
By-Products |
Bacterial |
|
TLC |
Yes |
No |
No |
No |
No |
|
HPLC |
Yes |
Yes |
No |
No |
No |
|
Spectrophotometric |
Yes |
No |
Yes |
No |
No |
|
Conductivity |
Yes |
Yes |
Yes |
Yes |
No |
|
TOC |
Yes |
Yes |
Yes |
Yes |
Yes |
|
1) An inactive substance that serves as the vehicle or medium for a drug or other active substance. |
|||||
HPLC/TLC
Although HPLC can be extremely accurate and quantitative, it tends to be highly selective in its approach, because each compound of interest interacts with the column differently based on polarity, pore size and stationary phase. Consequently, both HPLC/TCL are very useful for identifying known selective compounds, but fail to identify other types of contamination the analyst may not be aware of (foreign materials and bacteria). Additionally, TLC is not quantitative and cannot determine the quantity of contamination. Lastly, HPLC analysis requires significant operator skill to develop analytical methods. TOC analysis on the other hand is quite the opposite, requiring minimal operator skill for successful results.
Spectrophotometer
A spectrophotometer employs digestion in an enclosed glass chamber where carbon dioxide is oxidized using persulfate oxidation. The CO2 is dispersed into a solution that changes into carbonic acid and indicates the presence of carbon by changing color. This color change is proportional to the concentration of carbon in the sample. Because spectrophotometric analysis measures the color change for a reacted compound, it inherently has many interferences including sulfur, chlorine, manganese, calcium, copper, and iron, leading to unreliable results.
Conductivity
Conductivity measures the conductivity of the sample before and after oxidation; this difference yields the amount of TOC. The sample in the oxidation phase forms dissolved CO2 which acts as a weak acid, strengthening the conductivity of the sample. The amount of conductivity is proportional to the quantity of TOC in the sample. Hydrophobic gas permeation membranes are used to improve the accuracy of the conductivity method, allowing greater discrimination for dissolved CO2 over other chemical compounds.
Total Organic Carbon (TOC)
The TOC method can employ one of two different oxidation techniques:
- Chemical Oxidation (UV Persulfate [UVP] Systems)
- Catalyst Combustion Oxidation
Chemical oxidation transfers a sample aliquot to a UV reactor where oxidation is achieved through the combination of a chemical oxidizer, (usually sodium persulfate) and UV light. The oxidized carbon in the sample is converted to carbon dioxide (CO2) and swept through a detector that uses traditional non-dispersive infrared (NDIR) detection.
The second option, catalytic combustion oxidation, uses a catalyst to assist in the combustion of organic carbon into CO2. In these systems, the furnace-enclosed catalyst tube with sample is heated to 680 °C -1000 °C to oxidize carbon to CO2, through a combination of temperature, oxygen-rich environment (generally ultra-zero air or oxygen carrier gas) and catalyst. The CO2 is then swept to the NDIR detector.
Because the catalyst used in catalytic combustion systems can have carbon artifacts that create carbon background, UVP systems are recommended for the low-level analysis needed for PW and WFI applications. The only major interferences to the NDIR detection used in both systems are halogens and sulfur. To mitigate their effects, most TOC systems include a halogen scrubber and offer an optional sulfur scrubber.
21 CFR 11 Compatibility
The pharmaceutical industry is heavily regulated by government agencies to ensure consumer safety. One core regulation of contamination analysis is Code of Federal Regulation (CFR) Title 21 part 11 also known as 21 CFR 11. 21 CFR 11 mandates criteria required by the United States Food and Drug Administration (US FDA) for electronic recordkeeping and electronic signatures (ERES) in pharmaceutical validation software systems. The software used to collect data must be validated and meet the following key requirements:
- Must create an electronic record of collection, processing and analysis of data.
- Include electronic signatures that are legally binding.
- Track software activities (such as updates to methods).
- Validate that the system works properly.
- Document validation of the system.
The importance of meeting 21 CFR 11 requirements cannot be overstated. The software used to conduct the pharmaceutical cleaning validation must meet these requirements. The Fusion software is fully 21 CFR 11 compatible.
Fusion Pharmaceutical Methods
- High-Purity Water: ASTM D4779
- Cleaning Validation: USP <643> / EP 2.2.44 / JP 16 2.59
Relevant Application Notes are included below.
Teledyne Tekmar Fusion UV/Persulfate TOC Analyzer Application Notes
United States Pharmacopeia (USP) <643> Method “Bulk and Sterile Concentrations” provides guidelines and requirements for TOC analysis of cleaning validation, WFI and PW, and can be performed on Teledyne Tekmar’s Fusion UV/persulfate (chemical oxidation) TOC analyzer. The Fusion’s TekLink software is designed to meet 21 CFR 11 pharmaceutical requirements and includes electronic record and electronic signature (ERES), audit trails and user-account management features. Refer to the following application notes that demonstrate the Fusion’s ability to perform US<643>, WFI and PW validation and cleaning validation.

AN2204 - Fusion USP 643 Bulk and Sterile Water Testing (Updated USP <643> Effective May 1, 2021)
Objective
To explain the May 21, 2021 changes to United States Pharmacopeia (USP) Method <643> affecting the sterile water procedure, and demonstrate that the Teledyne Tekmar Fusion UV/Persulfate TOC analyzer can comply with these updates.
Background
USP <643> provides guidelines and requirements for TOC analysis of bulk water and packaged sterile water. This method includes a System Suitability Test that compares the recovery of a Standard
Solution (rs) of sucrose (a relatively easy compound to oxidize) to a System Suitability Solution (rss) of
1,4-benzoquinone (a difficult to oxidize compound). The response of Reagent Water (rw) is subtracted from the response of each solution, to yield corrected responses. The corrected responses are then compared and must be within 15% of each other to verify the system will sufficiently oxidize organic carbon within different compounds. The Response Efficiency must be between 85% and 115% for the results of the Bulk and Sterile Standards to meet USP <643> requirements using the equation in Figure 2.
|
% Response Efficiency = 100(rSS − rW)/(rS − rW) |
|
Where: · rSS = Instrument response to the System Suitability Solution (1, 4 Benzoquinone) · rW = Instrument response to the Reagent Water Control · rS = Instrument response to the Standard Solution (Sucrose) |
Figure 2: Equation from USP <643> to Calculate % Response Efficiency.
In 2007 Teledyne Tekmar published the application note, “Simplifying the Process: Automated USP 643 / EP 2.2.44 Purified Water and Water for Injection Testing Using a Next Generation TOC Analyzer” to demonstrate the Fusion’s ability to easily pass USP Method <643> requirements with 0.500 mg/L carbon standards. In 2017 we published USP Bulk & Sterile Water Using the Teledyne Tekmar Fusion UV/Persulfate TOC Analyzer in response to updates to USP Method <643>, including the Sterile Water concentration requirement of 8.000 ppmC.
May 1, 2021 Update to USP <643>
Prior to the May 01, 2021 update, packaged sterile water had a standardized limit and system suitability concentration of 8.000 ppmC. This concentration was applied to all sterile water regardless of container size. With the May 01, 2021 update, acceptance criteria for system suitability for all packaged sterile water has changed to be dependent on container size.
- This update does not affect the methodology and acceptance criteria for bulk water. The standard solution and system suitability solution used for bulk water remains at a concentration of
500 mg/L carbon.
Container size can directly influence the level of TOC that may leach from the container into the water. A small container will have a higher surface area to water ratio, consequently increasing the possibility of higher TOC levels from container leaching. Table II shows changes the USP has implemented due to higher leaching probability in relation to container size.
|
Table II TOC Limit Based on Container Volume |
||
|
Nominal Container Volume (mL) |
Limit 1 (mg/L of Carbon) |
Limit 2 (mg/L of Carbon) |
|
≤5 |
32.00 |
48.00 |
|
>5 and ≤100 |
24.00 |
36.00 |
|
>100 |
8.00 |
12.00 |
|
Note: Limit 2 concentrations are used to determine the system suitability requirements for the container volume being tested. |
||
Table III shows the instrumentation requirements for bulk water and sterile water along with the published limit of detection (LOD) capability of the Fusion UV/Persulfate TOC Analyzer.
|
Table III Instrument LOD Requirements and Fusion LOD Capability |
||
|
Bulk Water Requirement |
Sterile Water Requirement |
Fusion LOD Capability |
|
≤0.05 mg/L |
≤0.10 mg/L |
0.0002 mg/L |
Table IV shows the requirements for reagent water used for the procedures for bulk water and sterile water. Also listed is the practical quantification limit (PQL) of the Fusion UV/Persulfate TOC Analyzer.
|
Table IV Reagent Water Requirements and Fusion PQL Capability |
||
|
Bulk Water Requirement |
Sterile Water Requirement |
Fusion PQL Capability |
|
≤0.10 mg/L |
≤0.50 mg/L |
0.002 mg/L |
Instrument Methods
To demonstrate the Fusion’s ability to achieve the requirement for reagent water, the Fusion TekLink software’s default “TOC Pharmaceutical Water” method is required. This method uses a 9.0 mL sample volume per replicate which allows the Fusion to measure accurately at ppb levels.
- The Fusion’s default “TOC Pharmaceutical Water” method is also used for meeting the USP <643> bulk water system suitability requirements.
However, to analyze the “Limit 2” concentrations necessary to meet the method’s updated sterile water procedure for system suitability, a calibration with an upper limit of 50.0 mg/L is required. To achieve this upper limit, a dilution method must be created.
To create the required dilution method, the Fusion’s default “TOC Drinking Water” method was modified by simply changing the “Dilution” parameter to 1:5. This enables the Fusion to have an optimal upper calibration limit of 50.0 mg/L. The 1:5 dilution method parameters are shown in Figure 3.
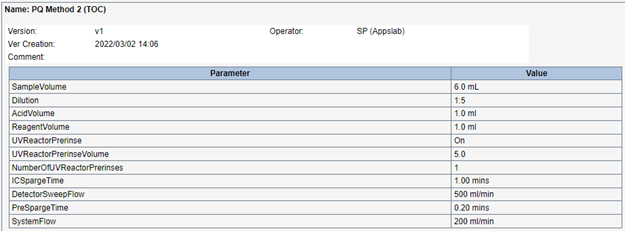
Figure 3: 1:5 Dilution Method.
1:5 Dilution Method Calibration Results
The results from the calibration established a coefficient of correlation (r2) of 0.99995. While a coefficient of correlation limit is not required by USP <643>, it is important to have an r2 value of >0.995 to attain accurate System Suitability results.
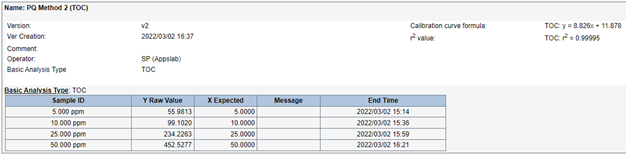
Figure 4: 1:5 Dilution Method Calibration Results.
Reagent Water Results
Reagent water was analyzed using a calibration created using the Fusion’s default “TOC Pharmaceutical Water” method. The r2 value for this calibration was 0.99997. The analysis result for reagent water was 0.0634 mg/L C. To pass the USP <643> requirement, the reagent water must be less than 0.500 mg/L C.
Sterile Water System Suitability Results
Sterile water concentrations required are represented by 12.0, 36.0 and 48.0 mg/L carbon for both sucrose and 1,4-benzoquinone standards. Standards were prepared according to USP <643> guidelines. For each concentration, the sucrose and 1,4-benzoquinone must have a response efficiency within 85-115% of each other using the equation shown in Figure 2. The accurate response efficiencies shown in Table V confirm how effectively the Fusion analyzes both easy and difficult to oxidize compounds at concentrations ranging up to 48.0 mg/L as required by the revised USP <643>.
|
Table V Sterile Water System Suitability Test Results |
||||
|
rW = Instrument Response to the Reagent Water Control (mg/L C) = |
0.0634 |
|||
|
rS = Instrument Response to the Standard Solution (Sucrose) |
||||
|
Concentration (mg/L C) |
12.00 |
36.00 |
48.00 |
|
|
Analysis Result (mg/L C) |
12.04 |
36.97 |
48.17 |
|
|
rSS = Instrument Response to the System Suitability Solution (1, 4 Benzoquinone) |
||||
|
Concentration (mg/L C) |
12.00 |
36.00 |
48.00 |
|
|
Analysis Result (mg/L C) |
11.75 |
35.96 |
46.49 |
|
|
Response Efficiency |
97.58 |
97.27 |
96.51 |
|
Conclusion
The Teledyne Tekmar Fusion UV/Persulfate TOC Analyzer successfully analyzed the revised USP <643> sterile water system suitability standard concentrations and was well within the requirement of 85% - 115%.
References
- United States Pharmacopeia <643> Total Organic Carbon [Revised: 01-May-2021].
Contact Us!
See how the Teledyne Tekmar Fusion UV/Persulfate Analyzer can help you comply with pharmaceutical and/or environmental standards. Contact a sales representative at 1.800.874.2004 or visit http://www.teledynetekmar.com/contact/sales-contacts. See genuine customer reviews of the Fusion at http://www.selectscience.net.
