
Has your laboratory been having issues obtaining helium for your environmental analysis? As helium supplies become scarcer and more expensive, you may have been seeking alternative carrier gases or ways to conserve helium without sacrificing system performance. This blog will explore ways to conserve helium during your purge and trap (P&T) analysis.
Perhaps your laboratory cannot use an alternative carrier gas for your Gas Chromatograph-Mass Spectrometer (GC-MS) because these alternatives come with trade-offs. Alternative carrier gases can often cause shifts in analyte retention time, separation issues, and require significant method parameter changes and validation times.
Recently, hydrogen has been a popular choice for an alternative carrier gas. The United States Environmental Protection Agency (US EPA) has updated methods allowing the use of hydrogen as the carrier gas. These updates include new 4-bromofluorobenzene (BFB) tune criteria, making it easier for hydrogen to pass criteria. Nitrogen as a carrier gas can have poor separation of analytes and have issues passing BFB tune criteria. There are not any updated US EPA drinking water or wastewater P&T methods using nitrogen as the carrier gas.
Since changing the carrier gas can lead to a lot of time-consuming activities, what are the other options for conserving helium during P&T analysis? Using an alternative purge gas or optimizing purge parameters could be beneficial for your laboratory. A great purge gas for volatile organic compound (VOC) analysis must be inert, meaning it cannot react with the VOCs or anything else in the sample. It cannot be combustible or unstable. It must be free of VOCs because you do not want to contribute to the total VOCs in the sample collected and create false positives or contaminate blanks.
Helium has always been the traditional purge gas of choice because of its superior inertness, stability, and compatibility with the GC-MS. A typical P&T concentrator uses up to 1200 milliliters (mL) of gas per active sample and up to 1200 mL/hour in standby mode. Choosing an alternative purge gas can lead to significant cost savings.
Teledyne Tekmar P&T allow for the use of helium or nitrogen as the purge gas. Nitrogen can be a desirable alternative purge gas because it has similar inertness to helium, low cost for cylinders, and it can be produced by generators. It often provides more than adequate purge efficiency for analytes under standard US EPA parameters.
Please see Figure 1 for a comparison of helium and nitrogen purge gases under US EPA 524.3 method conditions. Switching the purge gas to nitrogen is quite easy in our TekLink software. As soon as the purge gas is changed in the configuration section of the tools menu, it can leak check and calculate the flow rate based on nitrogen, thanks to the mass flow controller (MFC) and few to no significant changes to your P&T method have to occur.
Hydrogen is not an option for an alternative purge gas. During desorb pre-heat, the trap is sealed off and heats up to 250°C, which will cause an explosive environment by pressuring the hydrogen under the heat, causing a dangerous situation.
If switching the purge gas to nitrogen is not something your lab is able to do, there are certain helium-saving parameters you can implement in your method to help conserve. We can control the volume of gas that we use to purge by looking at the flow rate, meaning the amount of time we are purging and how fast. A nice balance needs to be found because an aggressive flow can cause pressure issues or push compounds of interest deep into the sorbent bed of the trap.
US EPA method 524.3 is used to analyze drinking water and allows for method flexibility to reduce sample processing time. This method allows you to optimize P&T parameters to maximize purge efficiency. Allowing shorter purge times with less flow creates an opportunity to conserve helium.
Table 1 displays the five key parameters that have flexibility with this method, and it shows the recommended and allowable range for those flow rates and times. This method does have strict quality control requirements, which is something to keep in mind when experimenting with various purge times and flow rates. But spending the time it takes to find a reduced total purge volume method could be beneficial by reducing sample cycle times and conserving pricey helium. Please see Figure 2 for a comparison of the minimum allowable method parameters from Table 1 compared to default water method parameters. These parameters could conserve your laboratory’s helium usage by up to 250 mL/minute total purge volume per sample.
Overall, if your laboratory can switch to nitrogen as a purge gas, it could reduce your operating costs against an uncertain helium supply, and you will not have to compromise chromatographic quality or invest development time into alternative carrier gas methods.
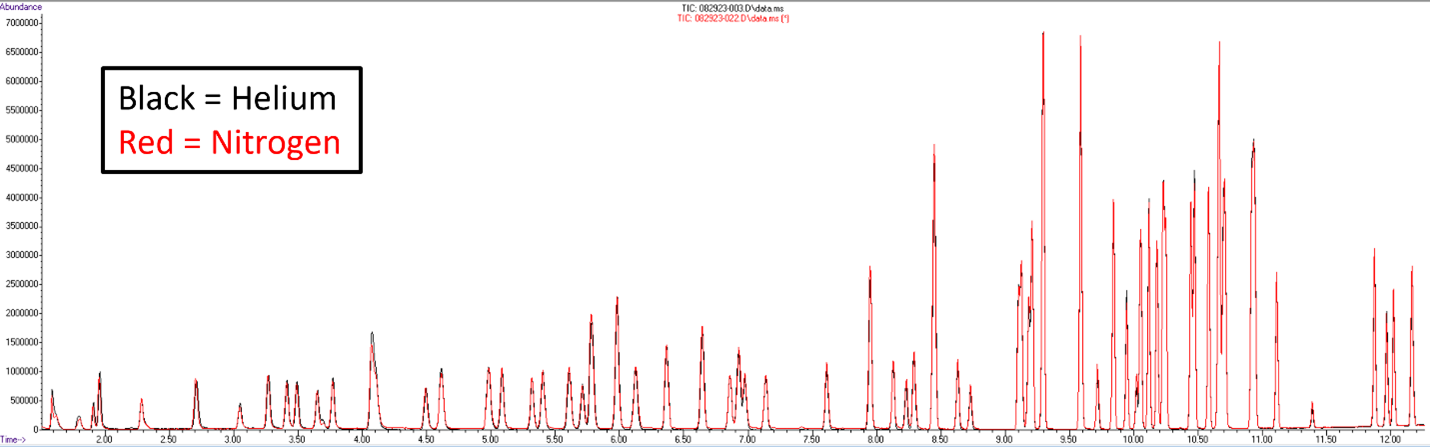
Figure 1: Purge gas comparison between helium and nitrogen under standard US EPA 524.3/4 method conditions.
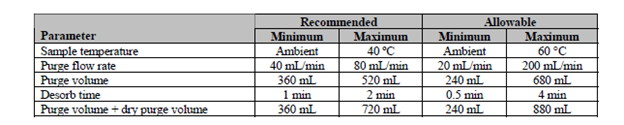
Table 1: Five key US EPA 524.3 method parameters that can be modified to optimize purge efficiency.
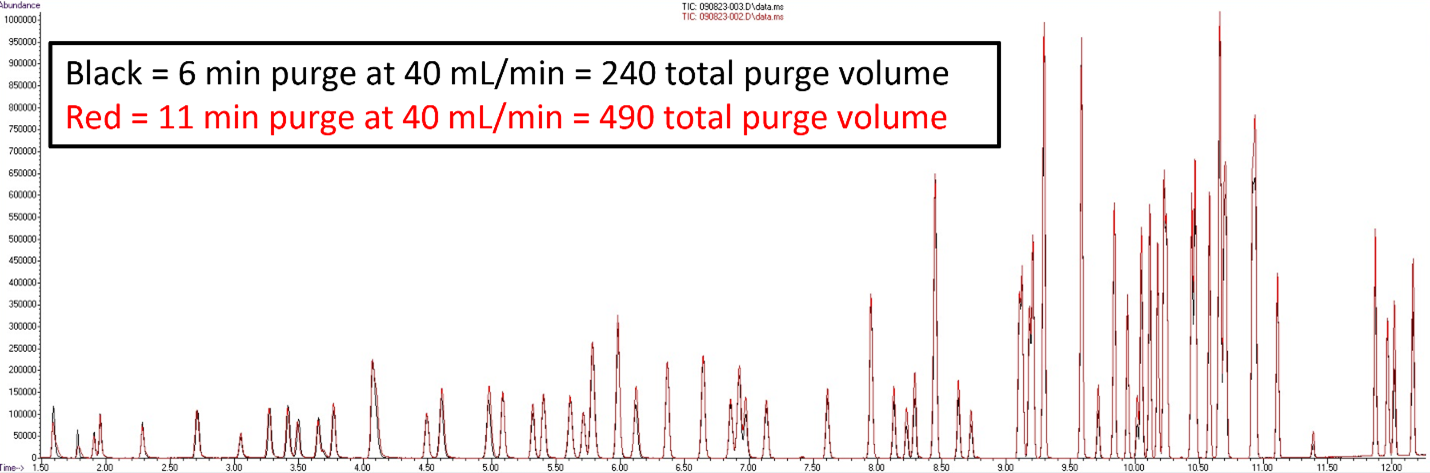
Figure 2: Minimum allowable US EPA 524.3 method parameters, 6-minute purge at 40 mL/minute for a total purge volume of 240 mL/minute compared to default water method parameters, 11-minute purge at 40 mL/minute and 0.5-minute dry purge at 100 mL/minute.
For more information, click the button below.
