Fundamentals of Purge & Trap
 Purge and Trap Background
Purge and Trap Background
When using a concentrator system, it is not essential to understand how it works. However, a good grasp of the fundamentals helps prevent problems and assists you when you are faced with tasks such as method development and troubleshooting. The purpose of this blog is to help you develop an understanding of how and why compounds are concentrated.
While gas chromatography is a powerful analytical tool, it does have several limitations. Many different techniques for a wide variety of samples have developed to overcome these limitations. The limitations, which P&T concentration is designed to overcome, include:
- Lack of Sensitivity:
GC detectors provide remarkable sensitivity. However, there are a number of areas where greater sensitivity is necessary. These include:
To provide an analytical system with comparable sensitivity, some method of concentration is required.
- Inability to Tolerate Water Injections:
Many GC columns and detectors do not perform well in the presence of water. Water may drastically reduce the lifetime of the column and adversely affect the detector performance.
- The Sample Must Be in Vapor or Vaporizable Form
Gas chromatography operates as an interaction between vapor and liquid phases. The sample must start out as a vapor. For this reason, there are many samples, such as pollutants in soil or flavors in solid food that cannot be directly introduced into a GC.
The ability to analyze VOCs is a vital part of environmental monitoring, outgassing studies, flavor or fragrance analysis, among others. P&T is a technique that separates the VOCs from a matrix. After separation, the VOCs are then concentrated and injected into the GC for separation.
Brief History
In the 1960s, P&T was used in the study of bodily fluids. In the mid-to-late 1970s, P&T became a technique that was well-known and widely applied due the need to monitor VOCs in drinking water. Using this technique, it was possible to detect sub-ppb level VOCs of a wide variety. Today, P&T is routinely applied in the environmental area for the analysis of VOCs in soil and water. The arrival of microprocessor-driven systems allows the concentrator to be more precise and automated, giving the operator more time for other projects.
Purge and Trap Operation Overview
A measured amount of sample is placed in a sealed vessel. The sample is purged with inert gas, causing VOCs to be swept out of the sample. The VOCs are retained in an analytical trap, which allows the purge gas to pass through to vent. The VOCs are then desorbed by heating the trap, injected into the GC by backflushing the trap with carrier gas, and separated and detected by normal GC operation.
Purging
In the previous section it states, ”The sample is purged with an inert gas, causing VOCs to be swept out of the sample.” This is a very simple-sounding way of describing what is in reality a rather complex process. Purging a sample to extract analytes is a gas extraction. There are many factors that affect the efficiency of this extraction. The amount of each compound purged is proportional to both its vapor pressure and its solubility in the sample. Both of these are in turn, affected by the sample temperature.
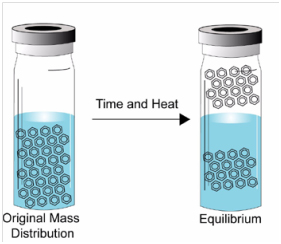
Consider the case of a sample sealed in a closed vial. Above the sample is a vapor space, which is usually referred to as the headspace. If you allow the sample sufficient time, VOCs in the sample will migrate into the vapor space. After a certain period of time, equilibrium will be established, and the concentration of the volatile compounds in each phase will be stabilized.
At this point a portion of the headspace can be removed and injected into the GC for analysis. The technique is known as Equilibrium Analysis or Static Headspace Analysis. The amount of material in the vapor phase will be proportional to the partial pressure of the component. The equation below describes the phase distribution of sample.
PT = P1 + P2 + P3 + ... + Pn = X1 P1o + X2 P2o + X3 P3o + ... + XnPno
where:
PT = total vapor pressure of system
P1, etc. = partial pressure of each compound
P1o, etc. = vapor pressures of the pure compounds
x1, etc. = mole fractions of each compound
In purging a sample, the system is no longer at equilibrium. This is because the VOCs that move into the vapor phase are constantly being removed by the purge gas. Under these circumstances, there is no migration of components from the vapor to liquid phase. This means that the partial pressure of any individual component above the sample at any time is essentially zero. This encourages even greater migration of the VOCs into the vapor phase, purging the sample more efficiently. Purging a sample for 10 minutes with helium (at a flow rate of 50 mL/min.) results in a more efficient extraction of volatiles than equilibrium, using 500 mL headspace. This purging technique is called Dynamic Headspace Analysis. For aqueous matrices, the increase in efficiency can be upwards of 100 fold, using dynamic versus static headspace analysis.
Extraction efficiency improves with an increase in sweep volume. Sweep volume, a function of sweep time and flow rate, is the amount of purge gas used to extract the analytes. Since the analytes are being trapped on a sorbent bed, there are limitations to the sweep times and flow rates that can be used. These limitations are determined by the compounds of interest in the sample and the sorbent material used in the trap.
Trapping and Adsorption
An analytical trap is a short gas chromatograph column. Compounds entering the trap will slowly elute with a measurable retention volume. Retention volume is the amount of purge gas that passes through the trap before elution of the analytes begins to occur.
The requirements of a trap are as follows:
- At low temperatures, it must retain the analytes while allowing oxygen and water to pass through unimpeded.
- Upon heating, it must release the analytes quickly and efficiently.
- When heated, it must show stability and not contribute to volatiles.
- It must operate without causing any catalytic reactions.
- It should have a reasonable price and lifetime.
At lower trap temperatures, retention volumes are high. At higher desorption temperatures, retention volumes are much smaller, allowing rapid transfer to the GC. In this context, the use of retention time is not correct. The correct parameter is retention volume.
When elution does occur, it is usually referred to as breakthrough, and the retention volume, at which breakthrough occurs, is often referred to as the breakthrough volume. Sorbent materials are usually chosen so that the breakthrough volume is high for analytes and low for water. Care must be taken that the sorbent chosen does not retain the analytes too strongly or efficient desorption may not be possible. Traps containing combinations of sorbents are often used to enhance performance.
The trap is packed with the weaker sorbent on top. The stronger sorbent is placed below the weaker sorbent. Less volatile analytes that are not effectively desorbed by the stronger sorbent are retained by the weaker sorbent.
Therefore, the less volatile analytes fail to reach the stronger sorbent. Only the more volatile analytes reach the stronger sorbent; and because of their volatility, these analytes can be efficiently desorbed. The desorption is carried out by backflushing the trap, ensuring that the heavier analytes never come in contact with the stronger sorbent.
Teledyne Tekmar developed the first commercial Purge and Trap concentrators in 1975, and today offers a comprehensive line of products, including the Stratum, which uses the Teledyne Hastings Mass Flow Controller to deliver unmatched precision and accuracy. Teledyne’s AQUATek 100 automates the sample preparation steps for analyzing liquid samples, while the Atomx Automated Sample Prep System combines an autosampler and Purge and Trap into a single instrument for analyzing VOCs in soils and water.
